Industry News
Research, Science & Manufacturer Updates
Researchers have identified a peptide in the mucus secreted by a South Indian frog that can kill certain types of flu viruses.
Results from a new study show that brain function can be restored by human blood plasma.
A study has found that patients with type 2 diabetes have a higher prevalence of chronic hepatitis B virus infection.
The Veterans Affairs (VA) Department has issued a rule that will allow all advanced-practice registered nurses, with the exception of certified registered nurse anesthetists, to practice to their full authority at VA facilities.
Results of a Phase II clinical trial ofPfizer’s preventive vaccine againstClostridium difficile (C. diff) showed the vaccine was safe and stimulated a C. diff targeted immune response.
A study has found that patients with fibromyalgia have a high prevalence of anti-thyroid-stimulating hormone receptor antibody.
A PhaseI/II study of nine patients with hemophilia B who underwent Spark Therapeutics’ SPK-9001 gene therapy are promising, despite two adverse autoimmune reactions.
A new study shows that individuals treated with intravenous immune globulin (IVIG) for Guillain-Barré syndrome are at increased risk of developing hypoalbuminemia (reduced albumin levels).
The Centers for Medicare andMedicaid Services has released the Use of New or Increased Pass Through Payments in Medicaid Managed Care final rule.
A new study shows that brains of people genetically inclined toward Alzheimer’s are likely to show abnormal immune reactions as early as seven years before the expected onset of the disease.
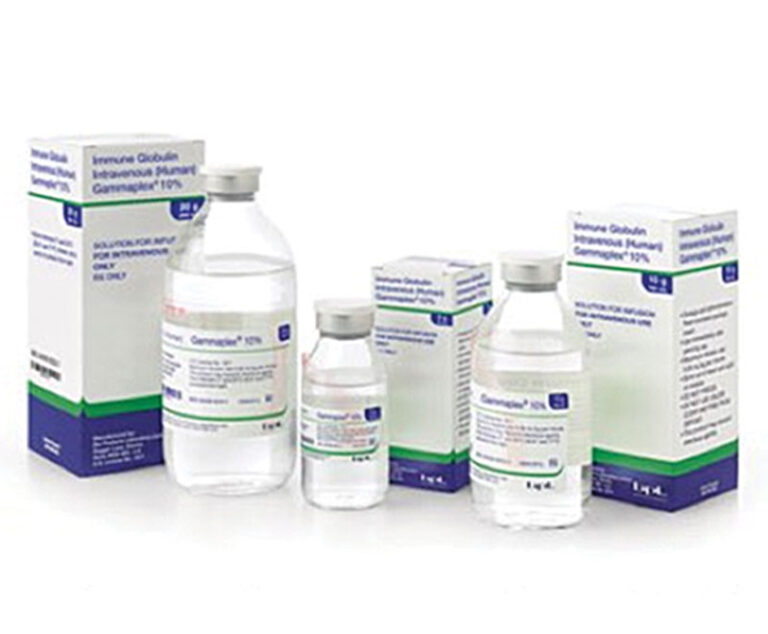
The U.S. Food and Drug Administration has approved Bio Products Laboratory’s Gammaplex 10% (immune globulin intravenous [human] 10% liquid) for the treatment of primary immunodeficiency (PI) and chronic immune thrombocytopenic purpura in adults.
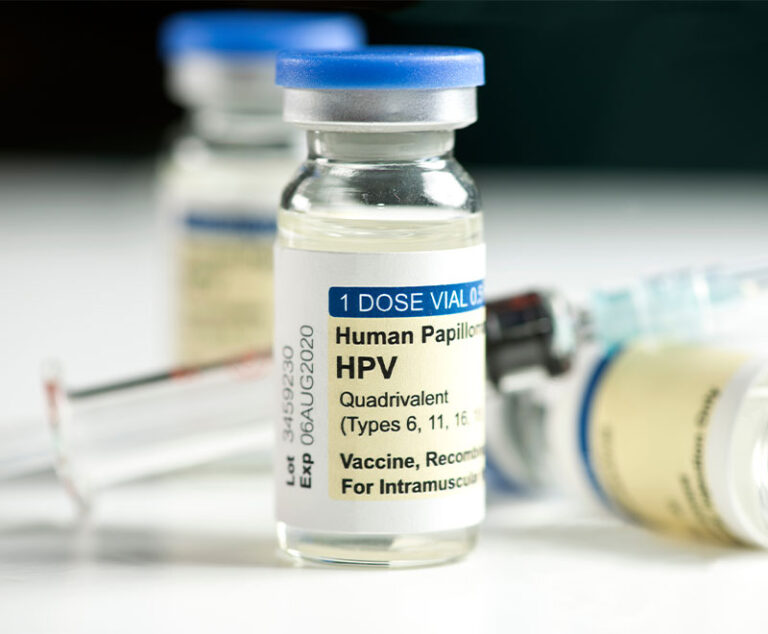
The Advisory Committee on Immunization Practices has changed its recommendation for the human papilloma virus from three doses to two doses of the vaccine prior to age 15.